Elon Musk Neuralink’s 1st Revolutionary Brain Chip Patient Can Effortlessly Control Computer Mouse With The Mind

The patient who first received Elon Musk’s Neuralink brain implant has fully recovered and, even more remarkably, can now move a computer mouse on a screen simply by thinking, without any physical movement. Elon Musk disclosed this on Monday during an X Spaces event hosted by journalist Katherine Brodsky. “Progress is good, and the patient […]
Multi-Omic Testing Reveals Biomarkers Tied to Lifestyle

Multi-omic microsampling analyzes biomarkers in tiny blood samples, enabling personalized tracking of health changes linked to lifestyle factors.
Scientists Develop Pill that Monitors Vital Signs

A revolutionary ingestible pill can remotely monitor vital signs like heart rate in real time. Human trials are planned for this futuristic capsule.
FDA Approves First Gene Therapies To Treat Patients With Sickle Cell Disease

The FDA approved the first two gene therapies, Casgevy and Lyfgenia, to treat sickle cell disease. These one-time treatments come with a hefty price tag.
Katalin Karikó and Drew Weissman, pioneers of the COVID-19 mRNA vaccine win the 2023 Nobel Prize in Physiology or Medicine

The 2023 Nobel Prize in Physiology or Medicine has been awarded to Katalin Karikó and Drew Weissman for their critical discoveries enabling mRNA vaccines against COVID-19. Their groundbreaking work laid the foundation for the Pfizer-BioNTech and Moderna vaccines used worldwide.
RCN Introduces New Definition of Nursing Highlighting Safety-Critical Role
The Royal College of Nursing has published a new definition of nursing that emphasizes the safety-critical, complex role of nurses as decision makers and problem solvers.
Elon Musk’s Neuralink Receives FDA Approval for Human Trials

Elon Musk’s brain-implant company, Neuralink, has received approval from the U.S. Food and Drug Administration (FDA) to conduct its first-in-human clinical trial, according to the company’s tweet on Friday. The trial will involve a small number of patients with severe paralysis who will be implanted with Neuralink’s brain-computer interface (BCI) device. The device is designed […]
FDA Approves First-ever Drug That Can Delay Onset of Type 1 Diabetes
Introduction Tzield (teplizumab-mzwv) has been approved by the US Food and Drug Administration to delay the onset of type 1 diabetes in people aged 8 years and older who are at high risk for developing the condition. What is type 1 diabetes? Type 1 diabetes is a disease that develops when the immune system attacks […]
Dr. Joe Hin Tjio; Research biologist who determined the correct number of human chromosomes
What are chromosomes? Chromosomes are threadlike structures consisting of protein and a single molecule of DNA that carry hereditary information in the form of genes. Each chromosome is made up of DNA tightly coiled many times around proteins called histones that support its structure. In plants and animals (including humans), chromosomes are found in the […]
Anaemia
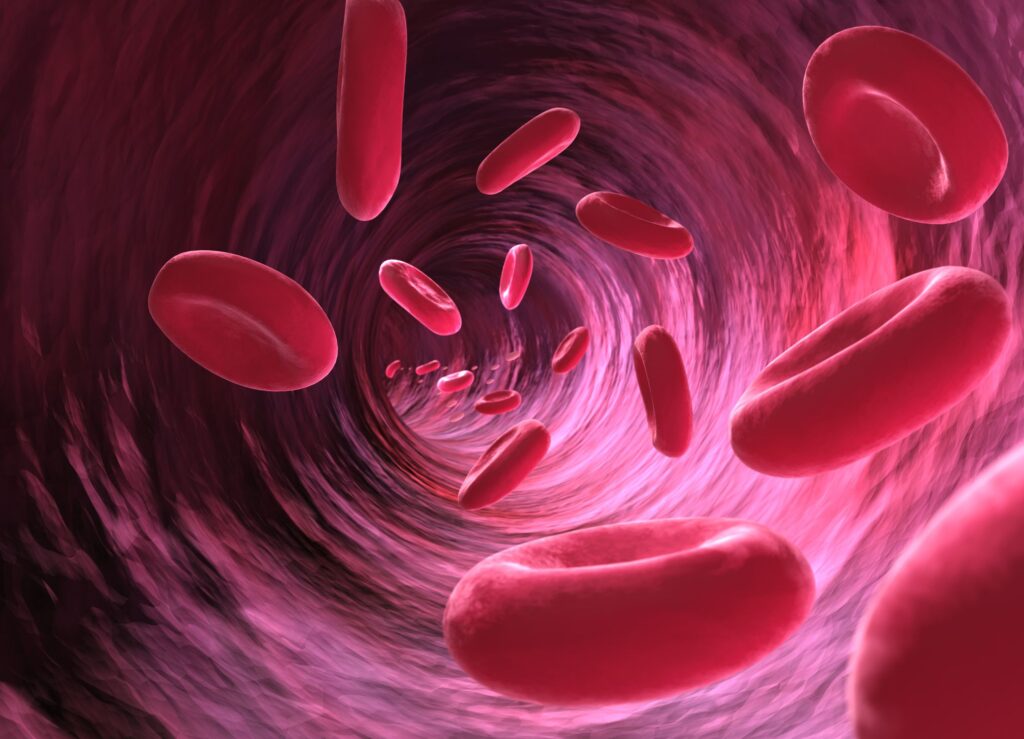
What is anaemia? Anaemia is a condition in which you lack enough healthy red blood cells to carry adequate oxygen to your body’s tissues. As in a routine blood test anaemia is reported by having a low hematocrit or haemoglobin levels. Anaemia can be mild or severe depending on its etiology and also the symptoms […]