Introduction
Tzield (teplizumab-mzwv) has been approved by the US Food and Drug Administration to delay the onset of type 1 diabetes in people aged 8 years and older who are at high risk for developing the condition.
What is type 1 diabetes?
Type 1 diabetes is a disease that develops when the immune system attacks and destroys the cells that make insulin. People who have been diagnosed with type 1 diabetes must monitor their blood sugar levels frequently throughout the day and must administer insulin shots or wear an insulin pump to survive. Although type 1 diabetes can manifest at any age, it is typically detected in children and young people. Although the majority of people with type 1 diabetes do not have a family history, having a parent, brother, or sister with the disease increases a person’s risk.
How the drug works
It is believed to work by reducing the body’s unintended attack on its own cells that make insulin. Protecting these cells is aimed at giving people more time until they require insulin to control their disease.
Tzield attaches to specific immune system cells and delays type 1 diabetes from progressing to stage 3. Tzield could inhibit the immune cells that attack the cells that produce insulin (pancreatic beta cells) while boosting the number of cells that help control the immune response.
Administration
Tzield is administered by intravenous infusion once daily for 14 consecutive days.
Side effects, Warnings and Precautions
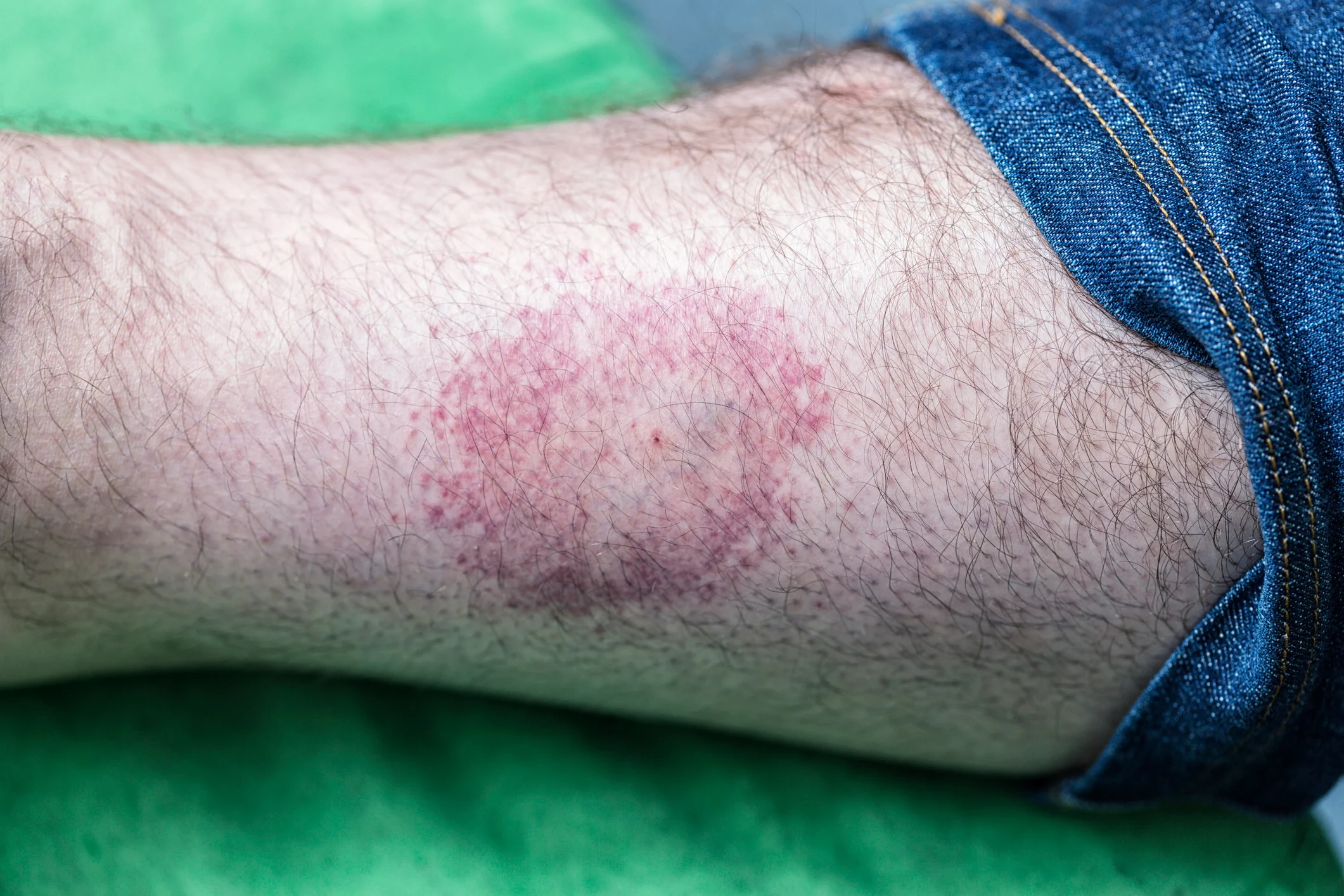
Tzield’s most common side effects include rash, headache, and decreased levels of certain white blood cells. The use of Tzield is accompanied by warnings and precautions, including the need to administer all age-appropriate vaccinations prior to starting Tzield and to avoid using live, inactivated, and mRNA vaccines concurrently with Tzield. Other risks and side effects include the need to premedicate and monitor for symptoms of Cytokine Release Syndrome; risk of serious infections; decreased levels of a type of white blood cell called lymphocytes; as well of risk of hypersensitivity reactions.